Introduction
The measurement of electrical conductivity in liquid or conductivity measurement as it is more commonly known is an extremely popular and important measurement. It is a measurement that is performed daily in a wide range of industries. The correct performance of the measurement is dependent on using high quality equipment, in particular a good sensor used in the correct manner and in the right environment. It is particularly important that appropriate conductivity standards are used, the sensor is calibrated correctly and in a timely manner and that the test is correctly controlled.
Let us start off by asking the question: What is electrical conductivity in liquid or as it is more commonly called electrical conductivity measurement.
Well, in simple terms, it is a measure of a solution’s ability to conduct electricity.
What is electrical conductivity?
In simple terms, it is a measure of a solution’s ability to conduct electricity.
Conductivity depends on the concentration of ions in solution, but does not identify the types of those ions. So, it gives an overall indication of inorganic content and it can be used as a single test for total ionic content of purified water. Conductivity reports rapidly a change of ionic concentration and this property combined with the fact that conductivity sensors can be configured in a rugged and robust manner, ensures that the test is ideal for in-process testing.
Basic Theory
The conductivity electrode and in particular the electrode plates are a critical part of the measurement. A known voltage is applied across the plates, as ions move between the electrode plates, producing an electric current and the conductivity meter measures this current. From the voltage and current values, the conductance can be calculated. However, precise measurement of the cell’s geometry is required to measure the conductivity.
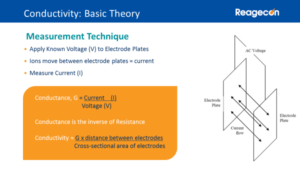
Rather than measuring the cell’s electrode dimensions, it is much easier to measure their effect on the measured value by testing the response to a Conductivity Standard:
Conductivity = Conductance, G x Cell Constant, k
This approach means that conductivity cells don’t need to be designed to permit measurement of their electrodes’ dimensions and nowadays appear with a variety of designs that are far more robust than the early parallel plates design.
Units of Measurement
Although, the base SI unit of measure of electrical conductivity is Siemens per metre, the most commonly used units are microsiemens / centimetre, followed by millisiemens / centimetre. This table gives guidance on the units of measure for conductivity, the symbols used to designate these units and how to convert one to the other.
For some industries and applications, it is electrical resistivity that is used, which is the inverse of electrical conductivity. Resistivity values expressed in megaOhm.centimetres can be calculated by dividing 1 by the conductivity value expressed in microsiemens/centimetre. Conductivity values expressed in microsiemens/centimetre can be calculated by dividing 1 by the resistance value expressed in Megaohm.centimetres. Resistivity is most commonly used for measurements of ultrapure water, where the purest water will have a conductivity of 0.055microsiems/centimetre at 25°C and a corresponding resistivity of 18.6 megaOhm-centimetres.
There is another unit of measurement of conductivity used in some countries which is known as micromhos/centimetre. The M H O part of this unit is just the word Ohm spelled in reverse and micromhos/centimetre are identical in value to microsiemens/centimetre.
Conductivity Sensor Range
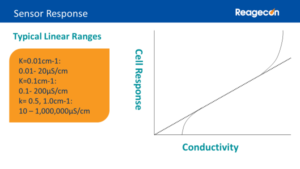
Conductivity sensors are available with a variety of different cell constants, most commonly ranging for k values of 0.01 to 10cm-1. Conductivity sensors have large linear measurement ranges. Below their linear measurement range, the sensors tend to under-report the conductivity value and above their linear measurement range they tend to over-report the conductivity value. Therefore, it is important to ensure that the samples’ conductivity range is covered by the sensor’s linear measurement range. This is achieved by selecting a cell with a k value from 0.01 to 0.1 for purified water and low conductivity applications and a cell with a k value from 0.5 to 10 for all other applications, so the entire measurement range can be covered with only 2 cells.
Temperature Effects
Temperature has a profound effect on the accuracy of conductivity measurement and not controlling or compensating for this, will lead to very significant measurement errors. Most conductivity sensors have inbuilt temperature sensors and most conductivity instruments have temperature compensation functions so that temperature effects can be accounted for. The temperature compensation function uses an algorithm to predict the conductivity value at a reference temperature value even if the measurement is performed at a different temperature. However, this approach assumes what the temperature effect is on the sample’s conductivity and assumptions are not perfect.
Standards
The calibration and control of the measurement is dependent on the availability and use of high-quality conductivity standards. Reagecon is the world’s largest producer of such standards and the only producer of low-level stable conductivity standards in an aqueous matrix. Such standards provide precision, accuracy, traceability, comparability, reproducibility and reduced uncertainty of measurement.
High quality standards can be used for calibration and control of the whole test system, but also to validate the test method, qualify the instrument and test the competency of the analyst. Such standards play a vital role as Reference Materials in the achievement and maintenance of ISO17025 and ISO17034 accreditations.
Calibration Standard Selection – Setting the K Constant
Setting the cell constant value is straightforward from testing response to a conductivity standard. The only requirement regarding selection of the Calibration Standard is that it must be in the cell’s linear response range. The Calibration Standard does not need to have a similar conductivity value to the test samples. Some instruments have automated calibration routines that require specific conductivity values for the calibration standard, all of which are available from Reagecon.
During calibration, the instrument measures the cell’s response and uses this to fix a response point against the value of the calibration standard. This point is signified by the dot on the graph. The instrument will also assume a second point at the origin, i.e. zero cell response corresponds to zero conductivity. The instrument then assumes that the cell’s response will be linear through these two points.
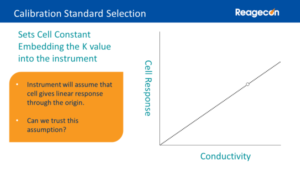
During the calibration process, the instrument makes several assumptions about the cell’s performance. Should these assumptions be trusted? It is better to test these assumptions through the use of Control Standards.
Control Standard Selection
Running Control Standards is an extremely important step in every area of analytical chemistry. Conductivity measurement is no exception to this. A valid Control Standard reading will be on or close to the expected response line of the measuring system. A valid Control Standard reading shows that the assumptions made by the instrument during calibration are valid, that the cell has a linear response and that the test method is valid. All of this leads to a very high level of confidence in the sample measurements, which is the ultimate objective when testing a sample. If Control Standard measurements are not made then there are no guarantees on the validity of the test and these measurements are made without any knowledge of whether they can be trusted.
The ideal Control Standard will have a value close to the expected conductivity value of the test samples. This proves that the measuring system and test method gives acceptable performance when measuring the test samples’ conductivity. If the test samples have a wide conductivity range then several Control Standards will be required to give confidence in the test samples’ measurements.
Reagecon Conductivity Standards
Reagecon is the market leader for Conductivity Standards. Reagecon provides a comprehensive range of Conductivity Standards that has the widest conductivity range and the highest number of conductivity values. This means that Reagecon Conductivity Standards cater for every required value for use as both Calibration and Control Standards. All of the Reagecon Conductivity Standards are fully traceable, have high specifications and have their values assigned by an ISO 17025 accredited test method.
Conductivity Standard Matrix
All of Reagecon’s Conductivity Standards have an aqueous matrix. As 99% of conductivity measurements are made on aqueous samples then this matches the samples’ matrix.
Some conductivity standards’ manufacturers produce non-aqueous conductivity standards – particularly for low conductivity values. These non-aqueous standards suffer from not matching the test sample matrix, they have low temperature dependency making accurate measurement onerous and they have hazard implications for transport, use and disposal. Reagecon’s all aqueous Conductivity Standards do not suffer from any of these drawbacks.
Stability of the Standard
All of Reagecon’s Conductivity Standards have fully validated shelf-lives. With the exception of the 1.3 microsiemens/centimetre standard, all of their expiry dates are independent of the date of first opening the bottle. This means that it is not necessary to record a reduced expiry date once the bottle has been opened.
Reagecon has a journal-published paper covering the stability of its lowest value conductivity standards. This paper only concentrates on the lowest conductivity values as this is the area where other manufacturers struggle to produce conductivity standards of proven stability and specification; but the study and findings reported in this paper are applicable to the entire range of Reagecon’s Conductivity Standards.
Effect of Temperature
We discussed earlier that there are significant temperature effects on conductivity measurement. To ensure that these effects can be accounted for, comprehensive temperature details are provided with all of Reagecon’s Conductivity Standards. The product labels include a detailed table of conductivity values at a wide range of temperatures and the coefficient to use for measurements taken using linear temperature compensation. This provides ease of use of Reagecon’s Conductivity Standards over a wide temperature range.
Certificate of Analysis
All of Reagecon’s products are provided to the customer with a certificate of analysis. This certificate states the nominal value of the standard, for example 10 microsiemens/centimetre at 25°C. It also contains the product number, lot number and expiry date. Most importantly it presents the exact measured value and the specification range for the product. The narrative of the certificate gives the test method, the actual NIST standard that the product is traceable to and the uncertainty of measurement attributable to the whole test system. Finally, in the case of conductivity standards the product is tested using an ISO17025 accredited test method. As with all of Reagecon’s certificates of analysis, the certificate is dated and signed.
Other Articles
Other articles that may be of interest:
